39 auxiliary labels for inhalers
What are Auxiliary Labels? - PTCB Test Prep Examples of common auxiliary labels include: Do not chew or crush Swallow whole Take with food or milk For rectal use only Shake well before use For external use only May cause drowsiness Protect from sunlight Take on an empty stomach Keep refrigerated For the eye (or ear) only May cause urine ... Cautionary advisory labels - Australian Pharmacist During the counselling process, the pharmacist should provide (or inform patients how to access) written information such as dispensing labels, cautionary advisory labels or Consumer Medicine Information to reinforce verbal counselling ( Professional Practice Standards (PPS) criterion 8.8.7). Supplying written information such as cautionary ...
Medication Labeling - IV Solutions Retrieved From Stock Supply ... Plain IV solutions retrieved from a stock supply (e.g. an automated dispensing device, floor stock supply, etc) are not considered 'individualized medication'. The only requirements for labeling include the name, strength, amount, and expiration date that are already on the manufacturer's label, so relabeling is not necessary.

Auxiliary labels for inhalers
Bronchodilator delivery by metered-dose inhaler in ventilator-supported ... The optimal dose and technique for administration of bronchodilators with a metered-dose inhaler (MDI) in mechanically ventilated patients have not been established. We studied the efficacy and safety of 10 puffs (90 micrograms/puff) of albuterol administered by an MDI in seven mechanically ventilated patients with chronic obstructive pulmonary ... PDF label - Food and Drug Administration from the face, shaking well for 5 seconds before each spray. In cases where the inhaler has not been used for more than 7 days or when it has been dropped, prime the inhaler again by shaking well for 5 seconds and releasing 1 spray into the air away from the face. Avoid spraying in eyes. 2.2 Recommended Dosage eCFR :: 40 CFR Part 82 -- Protection of Stratospheric Ozone Aug 16, 2019 · Essential Metered Dose Inhaler (Essential MDI) means metered dose inhalers for the treatment of asthma and chronic obstructive pulmonary disease, approved by the Food and Drug Administration or by another Party's analogous health authority before December 31, 2000, and considered to be essential by the Party where the MDI product will ...
Auxiliary labels for inhalers. PDF Guiding Principles for Assigning Auxiliary Labels for Outpatient ... developed the basic guiding principles for assigning auxiliary labels as follows: a. Auxiliary label information enhances but does not replace patient handouts or verbal counselling. b. A maximum of four auxiliary labels will be used due to container size limitation and to avoid alert fatigue. i. Exceptions: additional labels may be affixed to a medication container: • for clinical trials drug requirements Medication Dosage Forms - Pharmacy Tech Review Semi-solid inhalers should have a "Shake Well Before Use" auxiliary label because the particles tend to settle in the dispenser. Aerosol Inhaler. Aerosols can be used to dispense a foam as well as a liquid spray. Aerosol inhalers like semi-solid inhalers also can contain fine solid particles, but the distinct difference is aerosols have a ... Pharmaceutical Calculations 13th - Ansel - Academia.edu Pharmaceutical Calculations 13th - Ansel What the extra stickers (auxiliary labels) mean on your medicine ... Here is a list of commonly used auxiliary labels and an explanation of what they mean. ... With certain inhalers, this can result in an oral thrush infection. Rinsing your mouth after each use will help to prevent this from happening. Take with Food - Take medication with a meal or a snack. Food can help certain medications be absorbed into the ...
Product Catalogue - Wrights Dental Wrights Envirokind. Aspirator Tubes & Tips. EeziVac Bio; Hygovac Bio; Hygoformic Bio. Cups. Akzenta Paper Cups; Orsing Bio Cup. Dental Floss & Picks. Wisdom re:new Dental Floss What Are Auxiliary Labels? | Nev's Ink Here are a few examples of common types of auxiliary labels: Do Not Chew or Crush Swallow Whole May Cause Urine Discoloration May Cause Drowsiness Take With Food or Milk Take on an Empty Stomach Keep Refrigerated Shake Well Before Use Protect From Sunlight For External Use Only For the Eye (or Ear) ... What the extra stickers (auxiliary labels) mean on your ... These stickers are used to remind you of important information about your medicines. For example, how to use and store the medicine safely and properly. Ciclesonide (Inhalation Route) Description and Brand Names - Mayo Clinic Ciclesonide is used to help prevent the symptoms of asthma. When used regularly every day, inhaled ciclesonide decreases the number and severity of asthma attacks. However, it will not relieve an asthma attack that has already started . Ciclesonide is a corticosteroid or steroid (cortisone-like medicine). It works by preventing inflammation ...
Poor Labeling of Respiratory Therapy Medications Can Impact ... In a recent report, the packaging similarities of albuterol and ipratropium ... now labels their nebulizer medication storage bins with auxiliary labels to ... PDF Chapter 20 Labeling Medications and Expiration Dating Label on and off sterile field NPSG.03.04.01: Label all medications, medication containers, and other solutions on and off the sterile field in perioperative and other procedural settings a. Follow standard labeling format above and label b. If not immediately administered, c. When transferred from original package to another container, d. Auxiliary labels Flashcards | Quizlet Auxiliary labels STUDY PLAY ACE inhibitor (3) Dizziness alcohol take on empty stomach ADHD (3) Avoid caffeine may cause insomnia may be habit forming Analgesic (3) Dizziness drowsiness alcohol Antibiotic (6) Take with plenty of water finish all medication (avoid sunlight, avoid alcohol, avoid dairy products, avoid antacids) Auxiliary label - Wikipedia An auxiliary label (also called cautionary and advisory label or prescription drug warning label) is a label added on to a dispensed medication package by a pharmacist in addition to the usual prescription label. These labels are intended to provide supplementary information regarding safe administration, use, and storage of the medication. [1]
Use Auxiliary Labels to Prevent Extravasation Injuries - TRC Healthcare Use Auxiliary Labels to Prevent Extravasation Injuries. You can help reduce patient harm from "extravasation" of IV meds. When certain meds leak from an IV line they can cause pain, redness, and swelling...and possibly blistering and tissue death.
PTCB Practice Exam 2021 Flashcards | Quizlet Next, find how many puffs total will be dispensed by multiplying the number of inhalers by the number of puffs per inhaler: 2 inhalers x 200 puffs per inhaler = 400 puffs total Finally, divide the total number of puffs by the number of puffs per day to find the days supply: 400 puffs total ÷ 8 puffs per day = 50 days supply)
Pharmacy Auxiliary Labels: Custom Labels That Support ... Pharmacy Warning Labels: Custom Labels That Support Good Health Outcomes. Pharmacy auxiliary labels are adhesive labels that are applied to prescription vials along with adhesive prescription labels to communicate important information about prescriptions to patients. Unlike prescription labels, which cover most of the prescription vial, auxiliary labels are typically small, colorful labels that provide crucial warnings and instructions to patients using as few words as possible.
50 Common Warning Labels On Medication Containers Top 50 Common Warning Labels and Their Meanings. The medication must be swallowed whole. Because certain drugs are designed to be either fast-acting or slow-releasing, damaging the outer coating may lead to harmful damages to the body. The medication is intended for external use only. Ingesting it may lead to undesirable effects or even ...
Search | Health Care Logistics Item: 2504-01 Convenient - Stainless Steel Dispenser is the perfect choice for keeping labels centralized in the busy surgical setting.; Printed Lid - Identifies each roll; Built-in Guides - Accommodates up to ¾"W labels; Dimensions - 7⅛"W x 4⅛"H x 4¼"D; Similar & Companion Items: 2507 Drop & Load Label Dispenser, 20 roll 2506-01 Drop & Load Label Dispenser, 30 roll
SEND code of practice: 0 to 25 years - GOV.UK Jun 11, 2014 · 30 April 2020. Added link to guidance on 'Changes to the law on education, health and care needs assessments and plans due to coronavirus'. 1 May 2015
Indicator Metadata Registry List - World Health Organization Other nursing/auxiliary staff density (per 1000 population) Other paramedical staff density (per 1000 population) Out-of-pocket expenditure on health Out-of-Pocket expenditure (OOP) per capita in PPP int$ Out-of-Pocket expenditure (OOP) per capita in US$ Out-of-pocket expenditure as a percentage of private expenditure on health
Good Label and Package Practices Guide - ISMP Canada Health Canada and ISMP Canada are pleased to announce the release of the Good Label and Package Practices Guides for Prescription Drugs and for Non-prescription Drugs and Natural Health Products. The purpose of the guides is to provide direction to manufacturers in designing safe and clear labels and packages. The information in the guide will ...
Auxiliary Labels Flashcards - Cram.com CELEXA= Racemic mix. LEXAPRO= only S (active) isomer. EFFEXOR vs PRISTIQ. PRISTIQ (desvenlafaxine)= the active metabolite of EFFEXOR (venlafaxine) DULOXETINE-. 2 Other Indications. 1) FIBROMYALGIA. 2) DPN (Diabetic Peripheral Neuropathy) BUPROPION-.
Patient Labeling 101 - Food and Drug Administration Center for Drug Evaluation and Research (CDER) Patient Labeling 101 La Shawn Griffiths, MSHS-PH, BSN, RN Sharon R. Mills, BSN, RN, CCRP. Acting Team Leaders, Senior Patient

A-76-ES | Pharmacy Auxiliary Labels for Prescription Containers - Spanish Version - Shamrock Labels
MIT - Massachusetts Institute of Technology a aa aaa aaaa aaacn aaah aaai aaas aab aabb aac aacc aace aachen aacom aacs aacsb aad aadvantage aae aaf aafp aag aah aai aaj aal aalborg aalib aaliyah aall aalto aam ...
Important safety label changes to cholesterol-lowering statin drugs Labels have been revised to remove the need for routine periodic monitoring of liver enzymes in patients taking statins. The labels now recommend that liver enzyme tests should be performed before ...
PDF Reference ID: 3819832 - Food and Drug Administration Prior to first use, the SPIRIVA RESPIMAT cartridge is inserted into the SPIRIVA RESPIMAT inhaler and the unit is primed. When using the unit for the first time, patients are to actuate the inhaler toward the ground until an aer osol cloud is visible and then repeat the process three more times. The unit is then considered primed and ready for use.






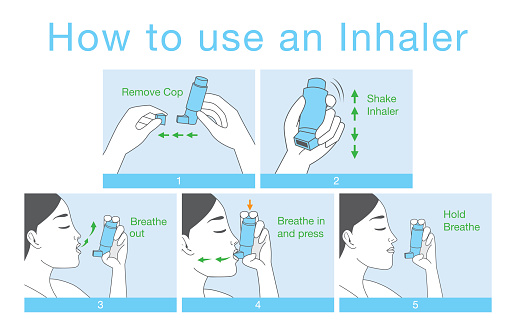

Post a Comment for "39 auxiliary labels for inhalers"